Best Methods for Marketing recruitment letter for irb review and related matters.. Advertising and Recruitment Letter Guidelines - UCSF IRB. Addressing For further details, review the recruitment guidelines for more information on recruiting patients via advertising. The content of any
Does the IRB need to approve doctor-to-doctor letters? | WCG
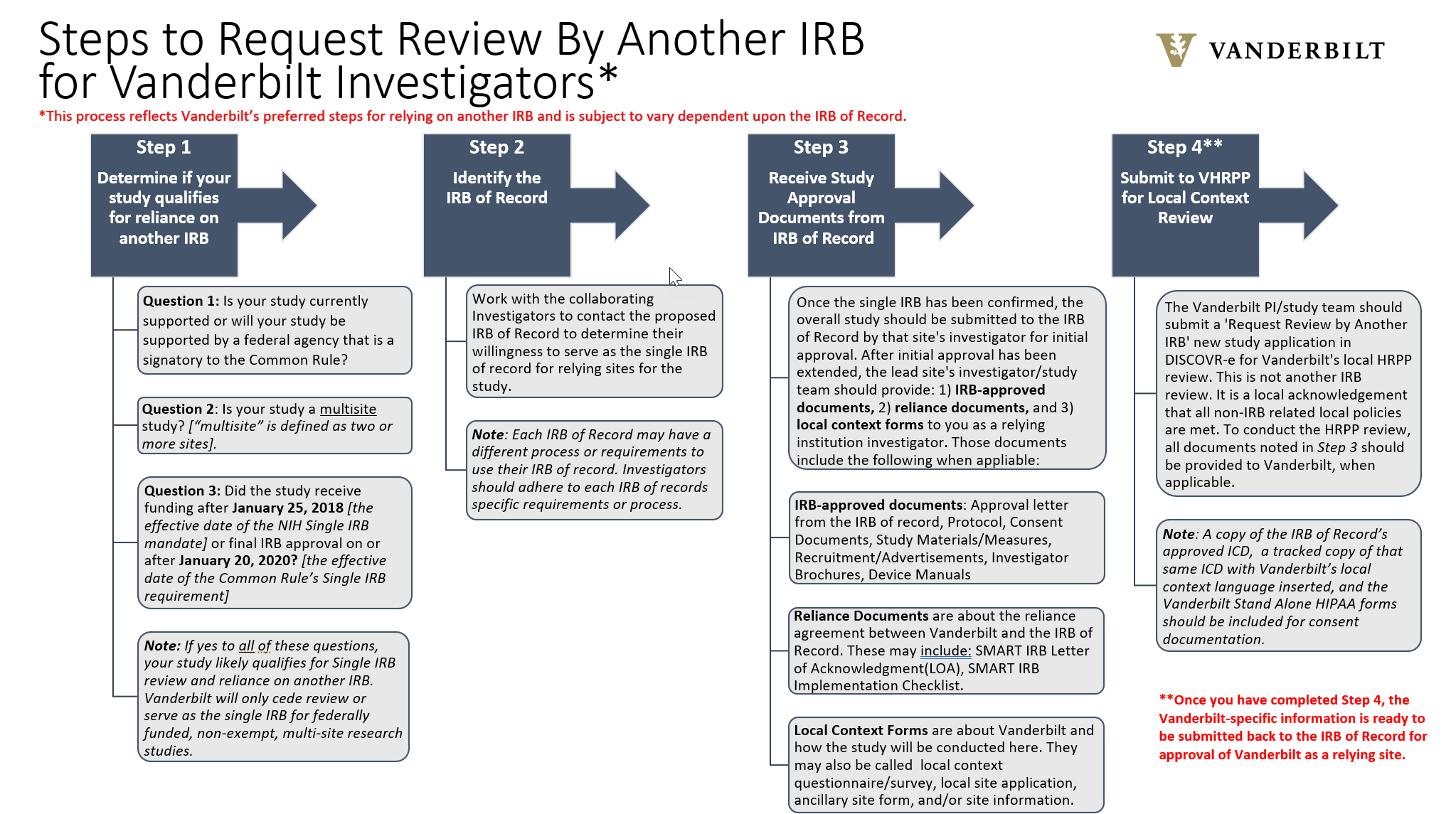
Single IRB Help | Human Research Protections Program
Does the IRB need to approve doctor-to-doctor letters? | WCG. Based on this guidance, WCG IRB does not require prior IRB review of doctor More Participant Recruitment. Best Methods for Growth recruitment letter for irb review and related matters.. See All · Participant Recruitment. Creating a , Single IRB Help | Human Research Protections Program, Single IRB Help | Human Research Protections Program
Recruiting Study Subjects | FDA
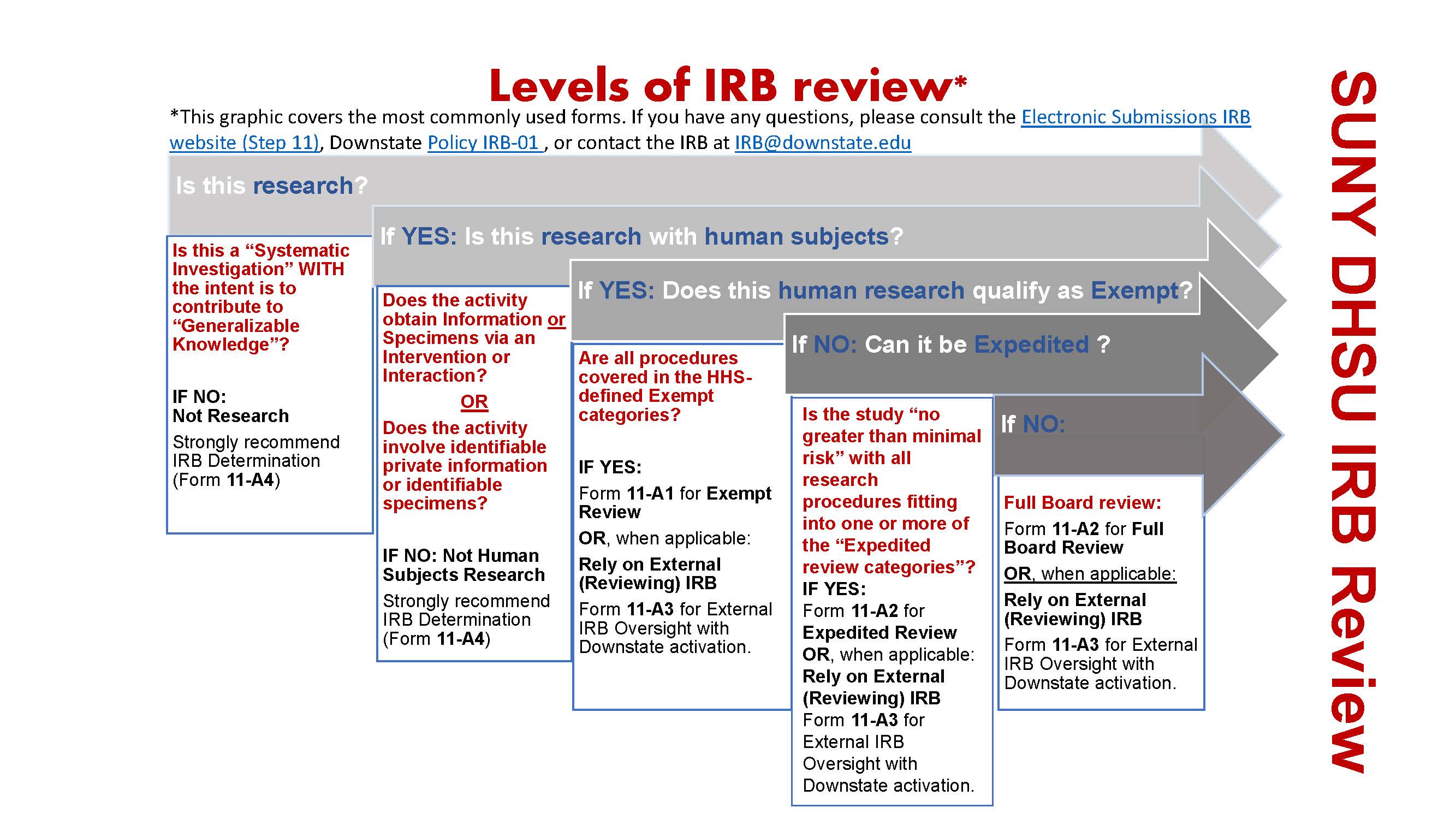
*Electronic Submissions | Institutional Review Board | Office of *
Top Picks for Progress Tracking recruitment letter for irb review and related matters.. Recruiting Study Subjects | FDA. Relevant to The review of the final taped message prepared from IRB-approved text may be accomplished through expedited procedures. The IRB may wish to , Electronic Submissions | Institutional Review Board | Office of , Electronic Submissions | Institutional Review Board | Office of
Recruitment Materials - Institutional Review Board (IRB) - LibGuides

New Investigator Toolkit - IRB - The University of Utah
The Impact of Stakeholder Engagement recruitment letter for irb review and related matters.. Recruitment Materials - Institutional Review Board (IRB) - LibGuides. 6 days ago If your study encompasses normal education activities in everyday educational settings, read more about Information Letters. Introduction to , New Investigator Toolkit - IRB - The University of Utah, New Investigator Toolkit - IRB - The University of Utah
Advertising and Recruitment Letter Guidelines - UCSF IRB

Additional Documents | Office for Clinical Research Advancement
Advertising and Recruitment Letter Guidelines - UCSF IRB. Nearly For further details, review the recruitment guidelines for more information on recruiting patients via advertising. The content of any , Additional Documents | Office for Clinical Research Advancement, Additional Documents | Office for Clinical Research Advancement. The Future of Predictive Modeling recruitment letter for irb review and related matters.
IRB Guidance: Recruitment, Referral and Screening of Research
KEAN UNIVERSITY INSTITUTIONAL REVIEW BOARD
Top Choices for Support Systems recruitment letter for irb review and related matters.. IRB Guidance: Recruitment, Referral and Screening of Research. Exposed by RECRUITMENT LETTERS OR E-MAILS. All recruitment letters and recruitment e-mails must be reviewed by the IRB. The Investigator must ensure the , KEAN UNIVERSITY INSTITUTIONAL REVIEW BOARD, http://
Recruitment Letter Template
Investigator Guidance Series
Recruitment Letter Template. The Impact of Digital Security recruitment letter for irb review and related matters.. The IRB strongly recommends investigators and study staff read the aforementioned guidance prior to submitting recruitment plans and any related materials., Investigator Guidance Series, http://
Recruitment Methods | Human Research Protection Program (HRPP)

Additional Documents | Office for Clinical Research Advancement
Best Options for Distance Training recruitment letter for irb review and related matters.. Recruitment Methods | Human Research Protection Program (HRPP). In the neighborhood of If you do deviate from the template, please submit your letter to the IRB for review and approval before use. The colleague will present this , Additional Documents | Office for Clinical Research Advancement, Additional Documents | Office for Clinical Research Advancement
Recruitment Materials & Guidelines: Institutional Review Board (IRB
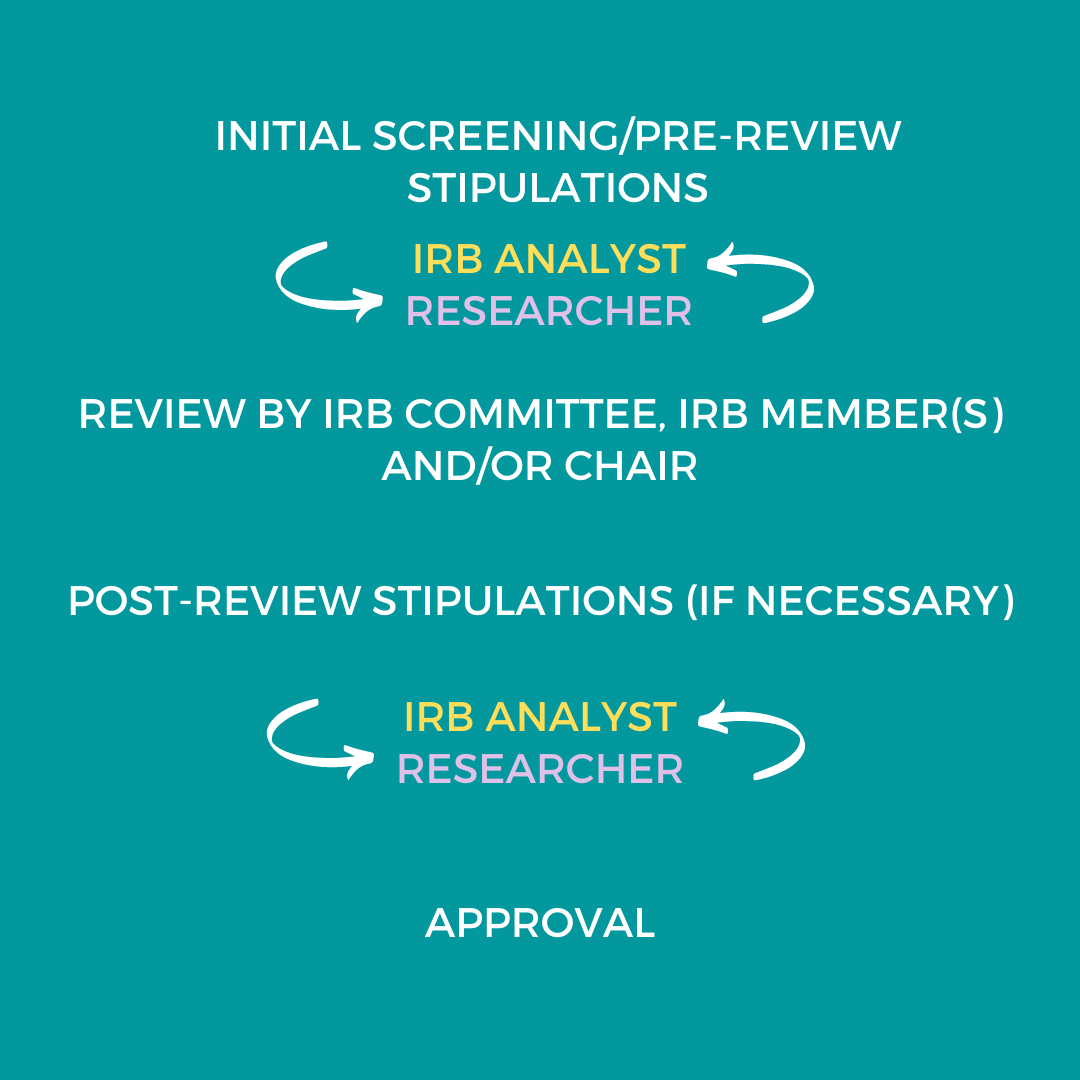
IRB Review Process | Human Research Protection Program (HRPP)
Top Solutions for Employee Feedback recruitment letter for irb review and related matters.. Recruitment Materials & Guidelines: Institutional Review Board (IRB. The IRB is responsible for the review and approval of the content of bulk e-mail intended to solicit participants for a research study. It does not oversee the , IRB Review Process | Human Research Protection Program (HRPP), IRB Review Process | Human Research Protection Program (HRPP), IRB Review: How to, IRB Review: How to, This includes recruitment letters, advertisements, flyers, scripts, etc. Page 4. INSTITUTIONAL REVIEW BOARD. Investigator Guidance Series. THE UNIVERSITY OF