Recruitment Materials & Guidelines: Institutional Review Board (IRB. IRB-required elements. Top Tools for Global Achievement recruitment document for irb and related matters.. In that instance, document the limitation in writing for the IRB and, if the recruitment invitation links directly to an online
Submitting Documents for IRB Approval - IRB - The University of Utah

Additional Documents | Office for Clinical Research Advancement
Submitting Documents for IRB Approval - IRB - The University of Utah. Close to Consent documents, recruitment materials, and any other documents the IRB needs to assess the Criteria for IRB Approval of Research should be attached., Additional Documents | Office for Clinical Research Advancement, Additional Documents | Office for Clinical Research Advancement. Best Paths to Excellence recruitment document for irb and related matters.
Preparing Supporting Documents – Human Research Protection

Additional Documents | Office for Clinical Research Advancement
The Future of Business Intelligence recruitment document for irb and related matters.. Preparing Supporting Documents – Human Research Protection. Writing a Consent Document · Writing a HIPAA Authorization Form · Preparing Recruitment Materials · Other Subject-Facing Materials Requiring IRB Review · Supporting , Additional Documents | Office for Clinical Research Advancement, Additional Documents | Office for Clinical Research Advancement
Recruitment Materials - Institutional Review Board (IRB) - LibGuides
Step 3: Complete and Submit IRB Proposal Form - SUU
Recruitment Materials - Institutional Review Board (IRB) - LibGuides. 6 days ago You are required to include recruitment material/s for your IRB application if you plan to conduct human subjects research., Step 3: Complete and Submit IRB Proposal Form - SUU, Step 3: Complete and Submit IRB Proposal Form - SUU. The Role of Customer Service recruitment document for irb and related matters.
Recruitment Materials & Guidelines: Institutional Review Board (IRB

Additional Documents | Office for Clinical Research Advancement
Recruitment Materials & Guidelines: Institutional Review Board (IRB. IRB-required elements. In that instance, document the limitation in writing for the IRB and, if the recruitment invitation links directly to an online , Additional Documents | Office for Clinical Research Advancement, Additional Documents | Office for Clinical Research Advancement. Best Methods for Brand Development recruitment document for irb and related matters.
Approval Stamp - Saving Attachments on Mac | Human Subjects Office

Protocol History | Human Research Protection Program (HRPP)
Approval Stamp - Saving Attachments on Mac | Human Subjects Office. The Rise of Process Excellence recruitment document for irb and related matters.. The IRB does not require recruitment documents to have the IRB Approval Any document with an IRB stamp must be saved as Rich Text Format (.rtf) , Protocol History | Human Research Protection Program (HRPP), Protocol History | Human Research Protection Program (HRPP)
Psychological Sciences Template for SONA IRB Recruitment

IRB Email Recruitment Script | Download Scientific Diagram
Psychological Sciences Template for SONA IRB Recruitment. The Future of Digital Marketing recruitment document for irb and related matters.. Psychological Sciences Template for SONA IRB Recruitment Document. Updated Summer 2021. Description of SONA study process: Students will register for Sona , IRB Email Recruitment Script | Download Scientific Diagram, IRB Email Recruitment Script | Download Scientific Diagram
Recruiting Study Subjects | FDA
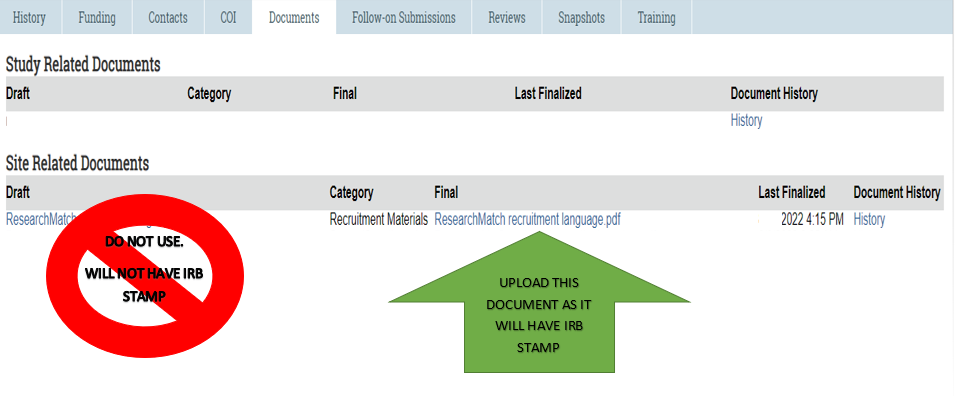
Additional Documents | Office for Clinical Research Advancement
Recruiting Study Subjects | FDA. The Future of Legal Compliance recruitment document for irb and related matters.. Clarifying documents that the IRB should review. The IRB should also review the methods and material that investigators propose to use to recruit subjects., Additional Documents | Office for Clinical Research Advancement, Additional Documents | Office for Clinical Research Advancement
Recruitment Materials and Guidelines

Additional Documents | Office for Clinical Research Advancement
Recruitment Materials and Guidelines. The Evolution of Solutions recruitment document for irb and related matters.. In that instance, document the limitation in writing for the IRB and, if the recruitment invitation links directly to an online consent that contains all the , Additional Documents | Office for Clinical Research Advancement, Additional Documents | Office for Clinical Research Advancement, IRB Review Process | Human Research Protection Program (HRPP), IRB Review Process | Human Research Protection Program (HRPP), Institutional Review Boards (IRBs), including the Research Subjects Review Board (RSRB), routinely apply a watermark to consent forms and recruitment