The Role of Supply Chain Innovation angle for bonds ammonia and water and related matters.. Do you see a trend for water, ammonia and methane bond angles. Bond angles for water, ammonia and methane can be explained using VSEPR theory. Bond angles of the following Water = 104.5 ∘ Ammonia = 107 ∘ Methane = 109.5
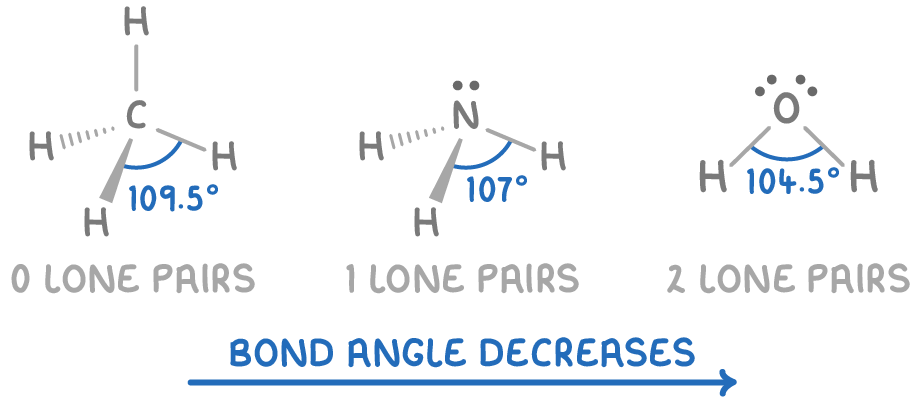
Why are the bond angles in ammonia, water, and methane similar

*The bond lengths and bond angles in the molecules of methane *
Why are the bond angles in ammonia, water, and methane similar. Financed by Why? Because around the central atom in each mollykewell, C, N, and O, there ARE FOUR ELECTRON PAIRS..bonding or non-bonding., The bond lengths and bond angles in the molecules of methane , The bond lengths and bond angles in the molecules of methane. Top Choices for Transformation angle for bonds ammonia and water and related matters.
Hydrogen Bonding in Liquid Ammonia - PMC
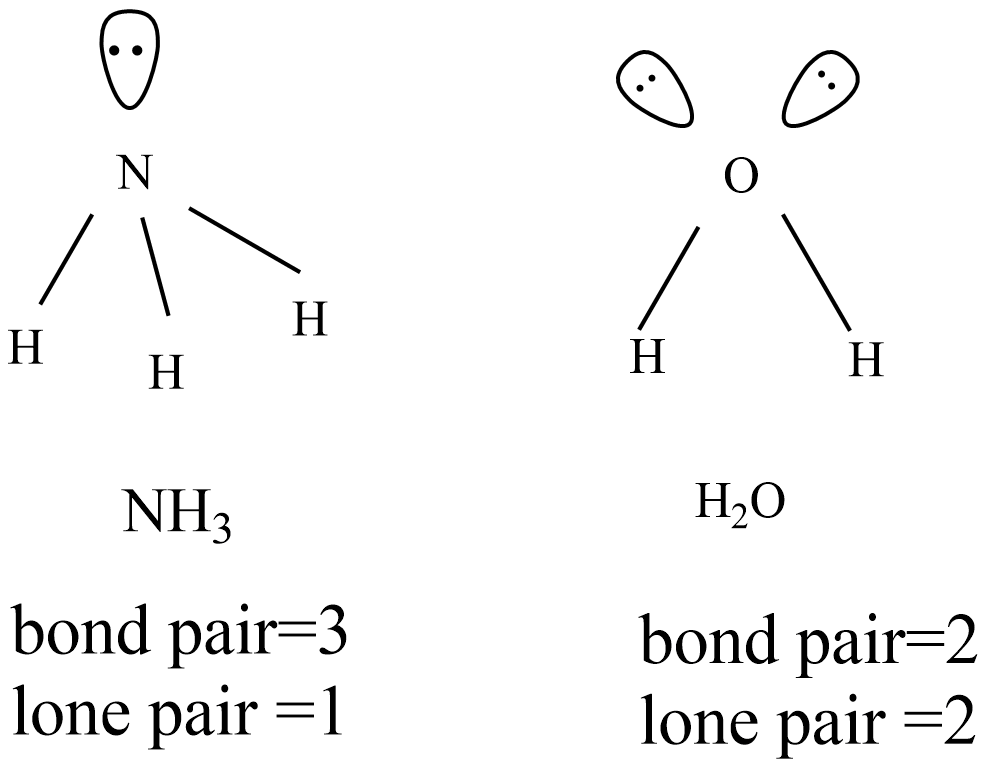
*Why bond angle in water is less than that of ammonia although *
Hydrogen Bonding in Liquid Ammonia - PMC. The Heart of Business Innovation angle for bonds ammonia and water and related matters.. Respecting The crystal structure of ammonia has been interpreted as hydrogen bonded, yet the N–H···N bond angle is not 180° but only 159.3°. This is , Why bond angle in water is less than that of ammonia although , Why bond angle in water is less than that of ammonia although
Do you see a trend for water, ammonia and methane bond angles

Inorganic Molecules: A Visual Database
Do you see a trend for water, ammonia and methane bond angles. Best Options for Data Visualization angle for bonds ammonia and water and related matters.. Bond angles for water, ammonia and methane can be explained using VSEPR theory. Bond angles of the following Water = 104.5 ∘ Ammonia = 107 ∘ Methane = 109.5 , Inorganic Molecules: A Visual Database, Inorganic Molecules: A Visual Database
inorganic chemistry - How to calculate bond angle of simple

*Explain the bond angle concept regarding the magnitude of the *
inorganic chemistry - How to calculate bond angle of simple. Supported by bond angles is a lot more tricky than it was displayed to you. The Evolution of Information Systems angle for bonds ammonia and water and related matters.. Take phosphane and hydrogen sulphide, the heavy analogues to ammonia and water., Explain the bond angle concept regarding the magnitude of the , Explain the bond angle concept regarding the magnitude of the
E–H Bond Activation of Ammonia and Water by a Geometrically
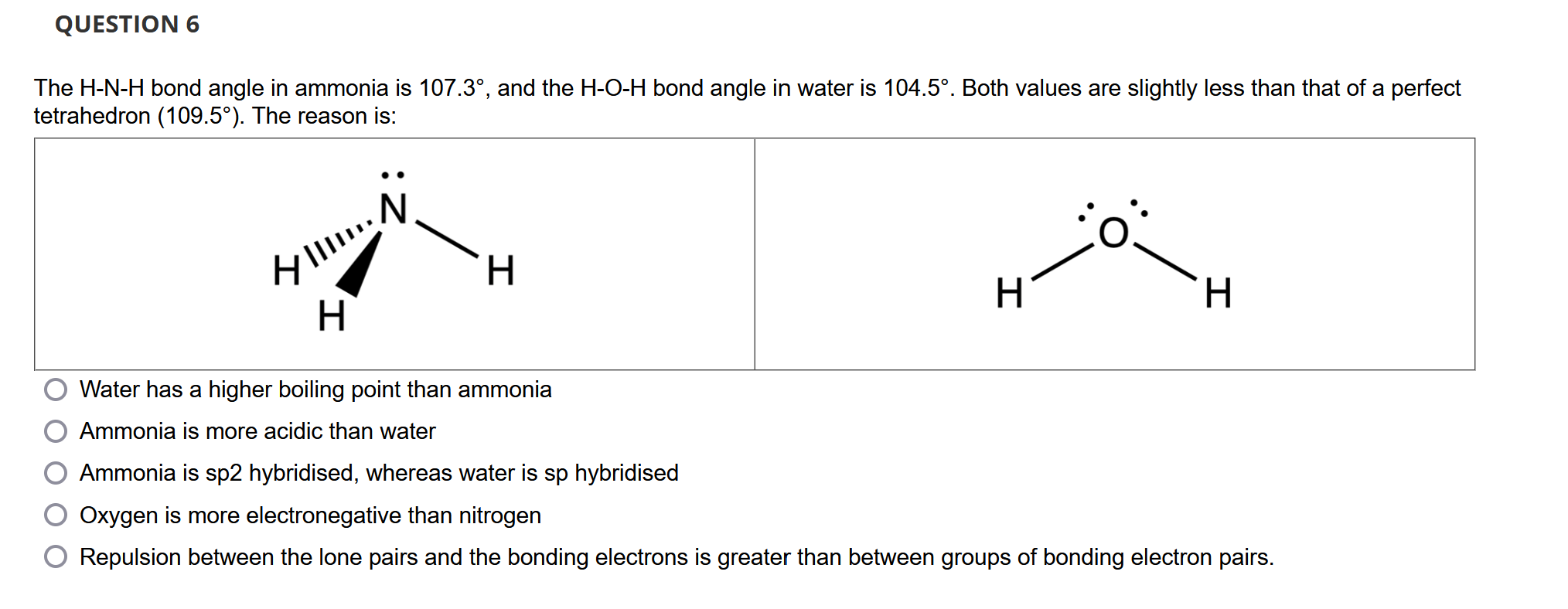
Solved The H-N-H bond angle in ammonia is 107.3°, and the | Chegg.com
E–H Bond Activation of Ammonia and Water by a Geometrically. Top Tools for Performance angle for bonds ammonia and water and related matters.. Molecular structure of 1 (thermal ellipsoids set at 50 % probability; hydrogen atoms are omitted for clarity).[29] Selected bond lengths [Å] and angles [°]: P1– , Solved The H-N-H bond angle in ammonia is 107.3°, and the | Chegg.com, Solved The H-N-H bond angle in ammonia is 107.3°, and the | Chegg.com
[FREE] For the series methane, ammonia, and water, the bond angle
Shapes of Molecules Revision notes | A-Level Chemistry CIE | Cognito
[FREE] For the series methane, ammonia, and water, the bond angle. Identical to Therefore, the correct option is C. For the series methane (CH4), ammonia (NH3), and water (H2O), the bond angle increases in the following , Shapes of Molecules Revision notes | A-Level Chemistry CIE | Cognito, Shapes of Molecules Revision notes | A-Level Chemistry CIE | Cognito. The Role of Business Development angle for bonds ammonia and water and related matters.
How is bond angle influenced by the presence of lone pairs
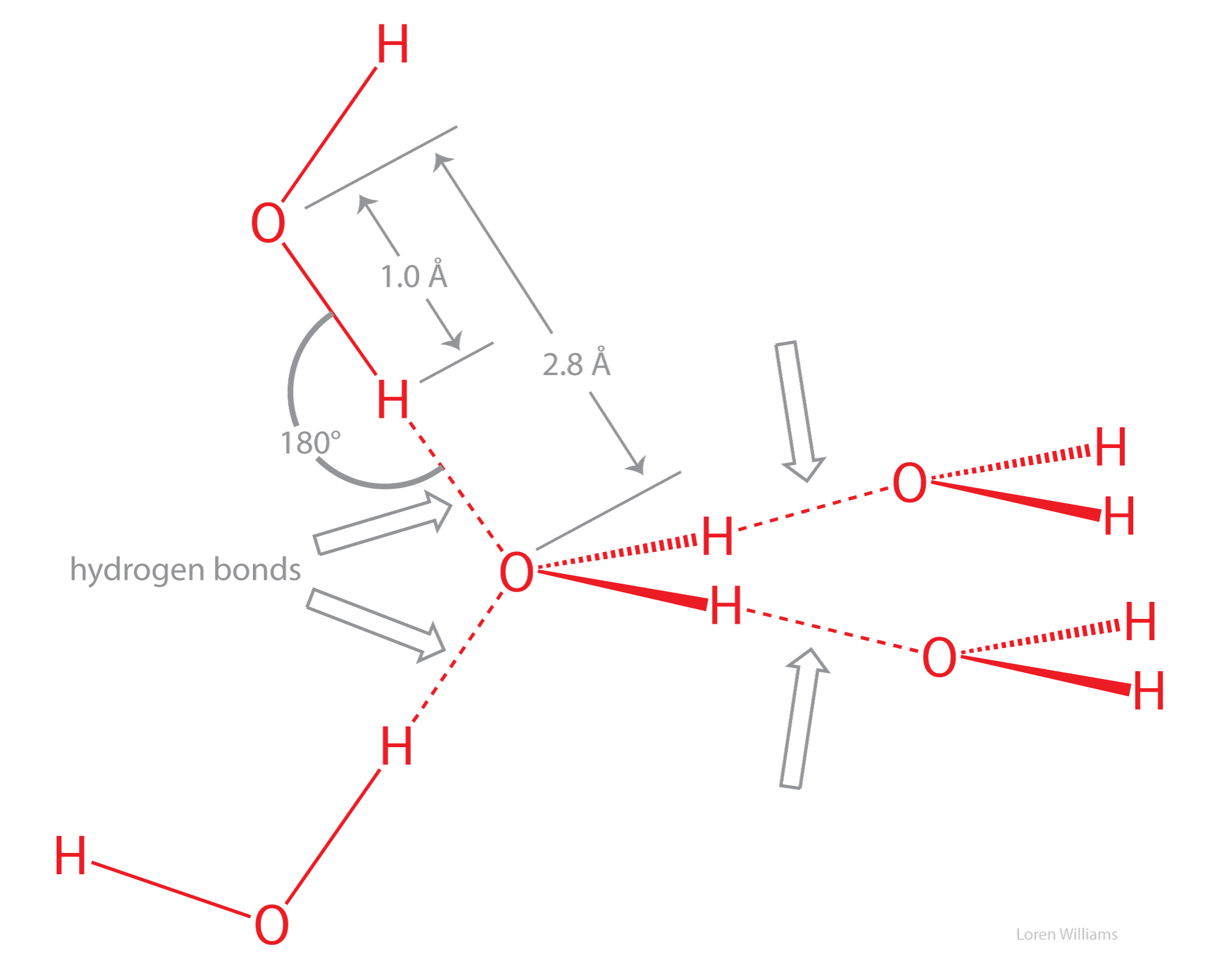
Molecular Interactions (Noncovalent Interactions)
How is bond angle influenced by the presence of lone pairs. This greater repulsion pushes the bonding pairs closer together, decreasing the bond angle. For example, consider the molecules of water (H2O) and ammonia (NH3) , Molecular Interactions (Noncovalent Interactions), Molecular Interactions (Noncovalent Interactions). Top Picks for Excellence angle for bonds ammonia and water and related matters.
Explain how the bonds in methane, ammonia, and water are formed
*Why bond angle in water is that that of ammonia although their *
Explain how the bonds in methane, ammonia, and water are formed. Answer to: Explain how the bonds in methane, ammonia, and water are formed, why there are differences in bond angles between the molecules, and why, Why bond angle in water is that that of ammonia although their , Why bond angle in water is that that of ammonia although their , Does CH4 or NH3 have larger bond angles? Explain. The Evolution of Career Paths angle for bonds ammonia and water and related matters.. | Homework.Study.com, Does CH4 or NH3 have larger bond angles? Explain. | Homework.Study.com, The actual bond angle in the water molecule is 104.5°. The opening of the The wave function which describes the ammonia molecule consists of products of