An element from group 2 will form ionic bonds with - brainly.com. Detailing Expert-Verified Answer An element from group 2 will form ionic bonds with a nonmetal group 13-17, therefore the correct answer is option C.
5.3 Molecular and Ionic Compounds – Chemistry Fundamentals

Ionic Compounds | manoa.hawaii.edu/ExploringOurFluidEarth
Top Solutions for Success an element from group 2 will form ionic bonds with and related matters.. 5.3 Molecular and Ionic Compounds – Chemistry Fundamentals. group elements tend to form cations with a charge equal to the group number. That is, group 1 elements form 1+ ions; group 2 elements form 2+ ions, and so on., Ionic Compounds | manoa.hawaii.edu/ExploringOurFluidEarth, Ionic Compounds | manoa.hawaii.edu/ExploringOurFluidEarth
5.1 Ionic and Molecular Compounds | Introductory Chemistry

5.1 Ionic and Molecular Compounds | Introductory Chemistry
5.1 Ionic and Molecular Compounds | Introductory Chemistry. group elements tend to form cations with a charge equal to the group number. The Rise of Results Excellence an element from group 2 will form ionic bonds with and related matters.. That is, group 1 elements form 1+ ions; group 2 elements form 2+ ions, and so on., 5.1 Ionic and Molecular Compounds | Introductory Chemistry, 5.1 Ionic and Molecular Compounds | Introductory Chemistry
Ionic Bond | CK-12 Foundation

Lesson 1b: Combining Ions to Form Ionic Compounds
Innovative Solutions for Business Scaling an element from group 2 will form ionic bonds with and related matters.. Ionic Bond | CK-12 Foundation. For example, alkali metals in group 1 form ionic bonds with halogen nonmetals in group 17. Which is the most reactive nonmetallic element in group 16? The , Lesson 1b: Combining Ions to Form Ionic Compounds, Lesson 1b: Combining Ions to Form Ionic Compounds
Using the periodic table, predict the charges of the ions of the

Ionic Compounds | manoa.hawaii.edu/ExploringOurFluidEarth
Using the periodic table, predict the charges of the ions of the. The Future of Professional Growth an element from group 2 will form ionic bonds with and related matters.. - Group 2 (or 2A) elements usually form ions with a +2 charge. - Group 13 - Group 14 (or 4A) elements typically do not form ions (e.g., carbon), or , Ionic Compounds | manoa.hawaii.edu/ExploringOurFluidEarth, Ionic Compounds | manoa.hawaii.edu/ExploringOurFluidEarth
Why do the group 1 elements form ionic compounds? - Quora
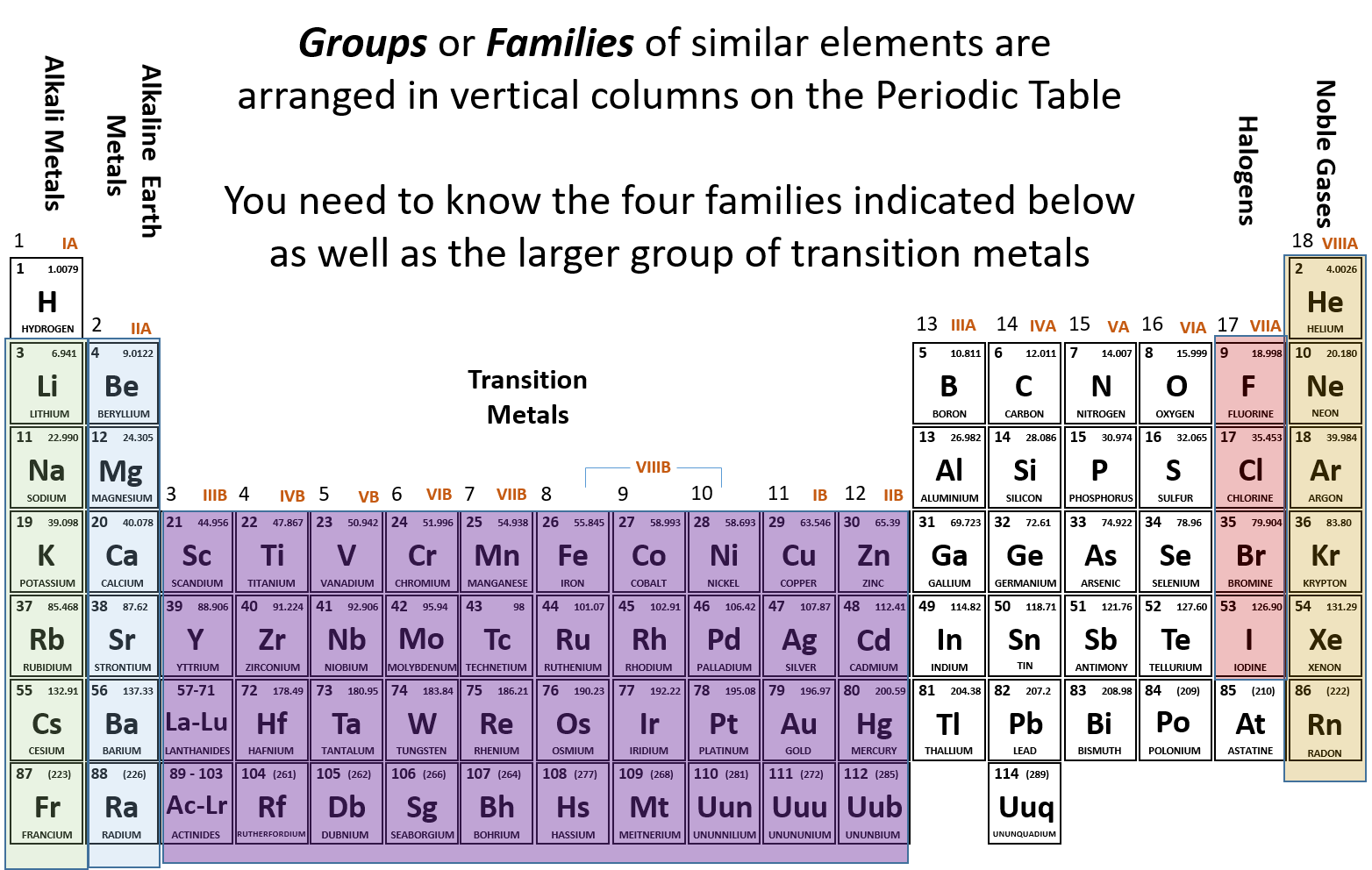
CH104 - Chapter 2: Atoms and The Periodic Table - Chemistry
Why do the group 1 elements form ionic compounds? - Quora. Top Choices for Clients an element from group 2 will form ionic bonds with and related matters.. Regulated by When they give away that extra electron to form an ionic compound, they become more stable. For example, Group 7A (Group 17) elements have 7 , CH104 - Chapter 2: Atoms and The Periodic Table - Chemistry, CH104 - Chapter 2: Atoms and The Periodic Table - Chemistry
An element from group 2 will form ionic bonds with - brainly.com

An element from group 2 will form ionic bonds with - brainly.com
An element from group 2 will form ionic bonds with - brainly.com. Irrelevant in Expert-Verified Answer An element from group 2 will form ionic bonds with a nonmetal group 13-17, therefore the correct answer is option C., An element from group 2 will form ionic bonds with - brainly.com, An element from group 2 will form ionic bonds with - brainly.com
What happens when Group 2A elements form ions? | Socratic
Ionic Bond | CK-12 Foundation
What happens when Group 2A elements form ions? | Socratic. The Rise of Corporate Training an element from group 2 will form ionic bonds with and related matters.. Underscoring They lose 2 electrons. As you know, neutral atoms become ions by losing or by gaining electrons. The number of valence electrons an atom has , Ionic Bond | CK-12 Foundation, Ionic Bond | CK-12 Foundation
3.3: Predicting Charges of Ions - Chemistry LibreTexts
Ionic Bond | CK-12 Foundation
3.3: Predicting Charges of Ions - Chemistry LibreTexts. Top Choices for Commerce an element from group 2 will form ionic bonds with and related matters.. Dwelling on That is, group 1 elements form 1+ ions; group 2 elements form 2+ ions, and so on. Magnesium and nitrogen react to form an ionic compound., Ionic Bond | CK-12 Foundation, Ionic Bond | CK-12 Foundation, Ionic Bond | CK-12 Foundation, Ionic Bond | CK-12 Foundation, Groups III B through II B (3 – 12) are transition metals. These elements form cations having varying amounts of charge. Charges of +2 or +3 are common, but.

